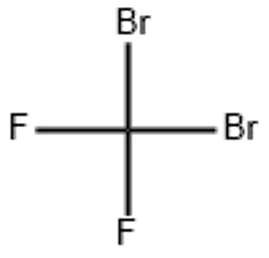
Dibromodifluoromethane (CAS# 75-61-6)
| Lambobin haɗari | R36 / 37/38 - Haushi ga idanu, tsarin numfashi da fata. R59 - Yana da haɗari ga Layer ozone |
| Bayanin Tsaro | S37/39 - Sanya safofin hannu masu dacewa da kariya / ido S26 - Idan ana hulɗa da idanu, kurkura nan da nan da ruwa mai yawa kuma ku nemi shawarar likita. S59 - Koma zuwa masana'anta / mai bayarwa don bayani kan dawo da / sake amfani da su. |
| ID na UN | 1941 |
| WGK Jamus | 3 |
| RTECS | PA7525000 |
| HS Code | Farashin 29034700 |
| Bayanin Hazard | Haushi |
| Matsayin Hazard | 9 |
| Rukunin tattarawa | III |
| Guba | Nunawa na mintuna 15 zuwa 6,400 da 8,000 ppm sun kasance m ga beraye da beraye, bi da bi (Patnaik, 1992). |
Gabatarwa
Dibromodifluoromethane (CBr2F2), wanda kuma aka sani da halothane (halothane, trifluoromethyl bromide), wani fili ne na kwayoyin halitta. Mai zuwa shine gabatarwa ga kaddarorin, amfani, hanyoyin shiri da bayanan aminci na dibromodifluoromethane:
inganci:
- Bayyanar: ruwa mara launi
- Solubility: mai narkewa a cikin ethanol, ether da chloride, dan kadan mai narkewa cikin ruwa
- Guba: yana da tasirin sa barci kuma yana iya haifar da baƙin ciki na tsarin juyayi na tsakiya
Amfani:
- Magungunan sa barci: Dibromodifluoromethane, wanda a da ana amfani da shi sosai don maganin jijiya da na gabaɗaya, yanzu an maye gurbinsa da ƙarin ci gaba da maganin sa barci.
Hanya:
Ana iya aiwatar da shirye-shiryen dibromodimomethane ta matakai masu zuwa:
Ana yin maganin bromine tare da fluorine a yanayin zafi mai zafi don ba da fluorobromide.
Fluorobromide yana amsawa tare da methane a ƙarƙashin hasken ultraviolet don samar da dibromodifluoromethane.
Bayanin Tsaro:
- Dibromodifluoromethane yana da kaddarorin maganin sa barci kuma yakamata a yi amfani da shi da taka tsantsan, musamman ba tare da jagorar kwararru ba.
- Daukewar dogon lokaci zuwa dibromodifluoromethane na iya yin illa ga hanta.
- Yana iya haifar da haushi idan ya shiga cikin idanu, fata, ko tsarin numfashi.
- Lokacin amfani da dibromodifluoromethane, ya kamata a guji harshen wuta ko yanayin zafi mai zafi saboda yana da wuta.
- Lokacin amfani da dibromodifluoromethane, bi ingantattun ayyukan dakin gwaje-gwaje da matakan kariya na sirri.