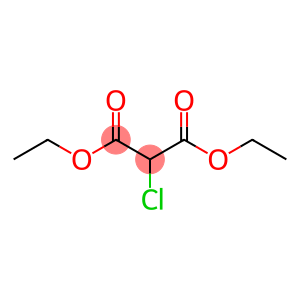
diethyl chloromalonate (CAS#14064-10-9)
| Alamomin haɗari | C - Mai lalacewa |
| Lambobin haɗari | R34 - Yana haifar da konewa R36 / 37 - Hannun idanu da tsarin numfashi. |
| Bayanin Tsaro | S26 - Idan ana hulɗa da idanu, kurkura nan da nan da ruwa mai yawa kuma ku nemi shawarar likita. S27 – Cire duk gurbatattun tufafi nan da nan. S28 - Bayan haɗuwa da fata, wanke nan da nan da sabulu-suds mai yawa. S36 / 37/39 - Sanya tufafin kariya masu dacewa, safar hannu da kariya / ido / fuska. S45 - Idan akwai haɗari ko kuma idan kun ji rashin lafiya, nemi shawarar likita nan da nan (nuna alamar a duk lokacin da zai yiwu.) |
| ID na UN | UN 3265 8/PG 2 |
| WGK Jamus | 3 |
| HS Code | 29171990 |
| Matsayin Hazard | 8 |
| Rukunin tattarawa | III |
Gabatarwa
Diethyl chloromalonate (kuma aka sani da DPC). Mai zuwa shine gabatarwa ga kaddarorin, amfani, hanyoyin shiri da bayanan aminci na diethyl chloromalonate:
1. Hali:
- Bayyanar: Diethyl chloromalonate ruwa ne mara launi.
- Solubility: Yana da narkewa a cikin mafi yawan kaushi na halitta, kamar su alcohols, ethers, da aromatic hydrocarbons, amma dan kadan mai narkewa a cikin ruwa.
- Kwanciyar hankali: Yana da ɗan kwanciyar hankali ga haske da zafi, amma yana iya samar da iskar hydrogen chloride mai guba a yanayin zafi mai zafi ko buɗe wuta.
2. Amfani:
- A matsayin sauran ƙarfi: Diethyl chloromalonate za a iya amfani dashi azaman mai narkewa, musamman a cikin ƙwayoyin halitta don narke da amsa abubuwan da ke tattare da kwayoyin halitta.
- Kemikal kira: Yana da reagent da aka saba amfani dashi don haɓakar esters, amides, da sauran mahaɗan kwayoyin halitta.
3. Hanya:
- Diethyl chloromalonate za a iya samu ta hanyar amsawar diethyl malonate tare da hydrogen chloride. Yanayin halayen gabaɗaya yana cikin zafin jiki, ana shigar da iskar hydrogen chloride a cikin diethyl malonate, kuma ana ƙara mai kara kuzari don haɓaka halayen.
- Ma'anar amsawa: CH3CH2COOCH2CH3 + HCl → ClCH2COOCH2CH3 + H2O
4. Bayanin Tsaro:
- Diethyl chloromalonate yana da ƙamshi mai ƙamshi kuma yana iya haifar da haushi ga fata, idanu, da fili na numfashi.
- Ruwa ne mai ƙonewa wanda ke buƙatar adana shi a wuri mai sanyi mai kyau kuma mai nisa daga tushen wuta da buɗe wuta.
- Yakamata a sanya kayan kariya da suka dace kamar safar hannu, tabarau, da tufafin kariya yayin sarrafawa.